
BSI CTMS
State-of-the-art Clinical Trial Management System with integrated eTMF, eMVR & Trial Supply Management for pharma, CROs and hospitals.
What is BSI CTMS?
BSI CTMS is a state-of-the-art Clinical Trial Management System with integrated eTMF, eMVR & TSM for Pharma, Biotech, MedTech, CROs & SMOs. A user-friendly interface with a hierarchical navigation tree allows you to oversee all aspects of your studies, from regulatory approvals to recruitment, from payments and contracts to monitoring and inventory. Plus, the mission control dashboard and alert notification system ensure you ll never miss a deadline! - 21 CFR Part 11 & EU Annex 11 compliant.
Screenshots
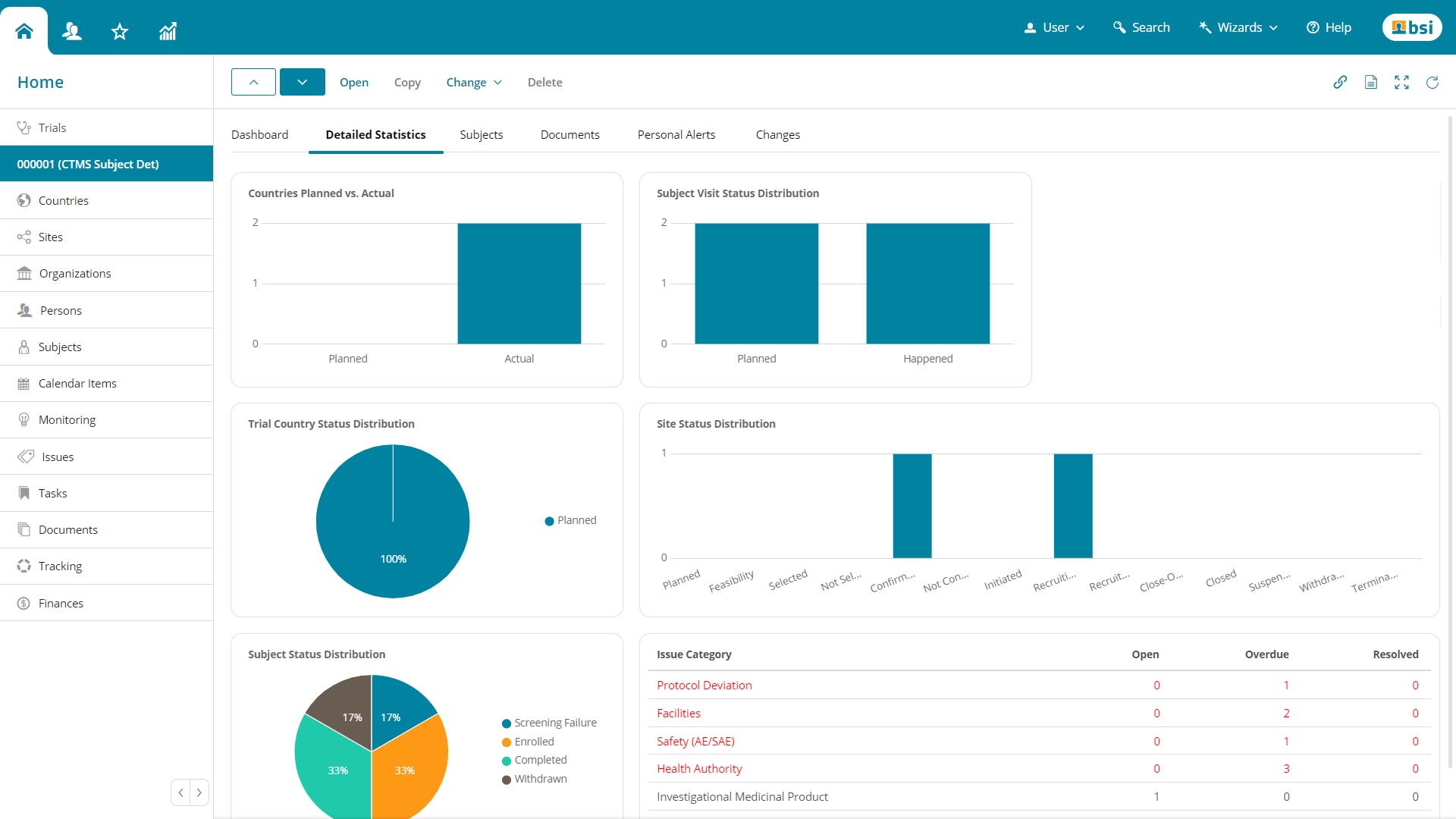

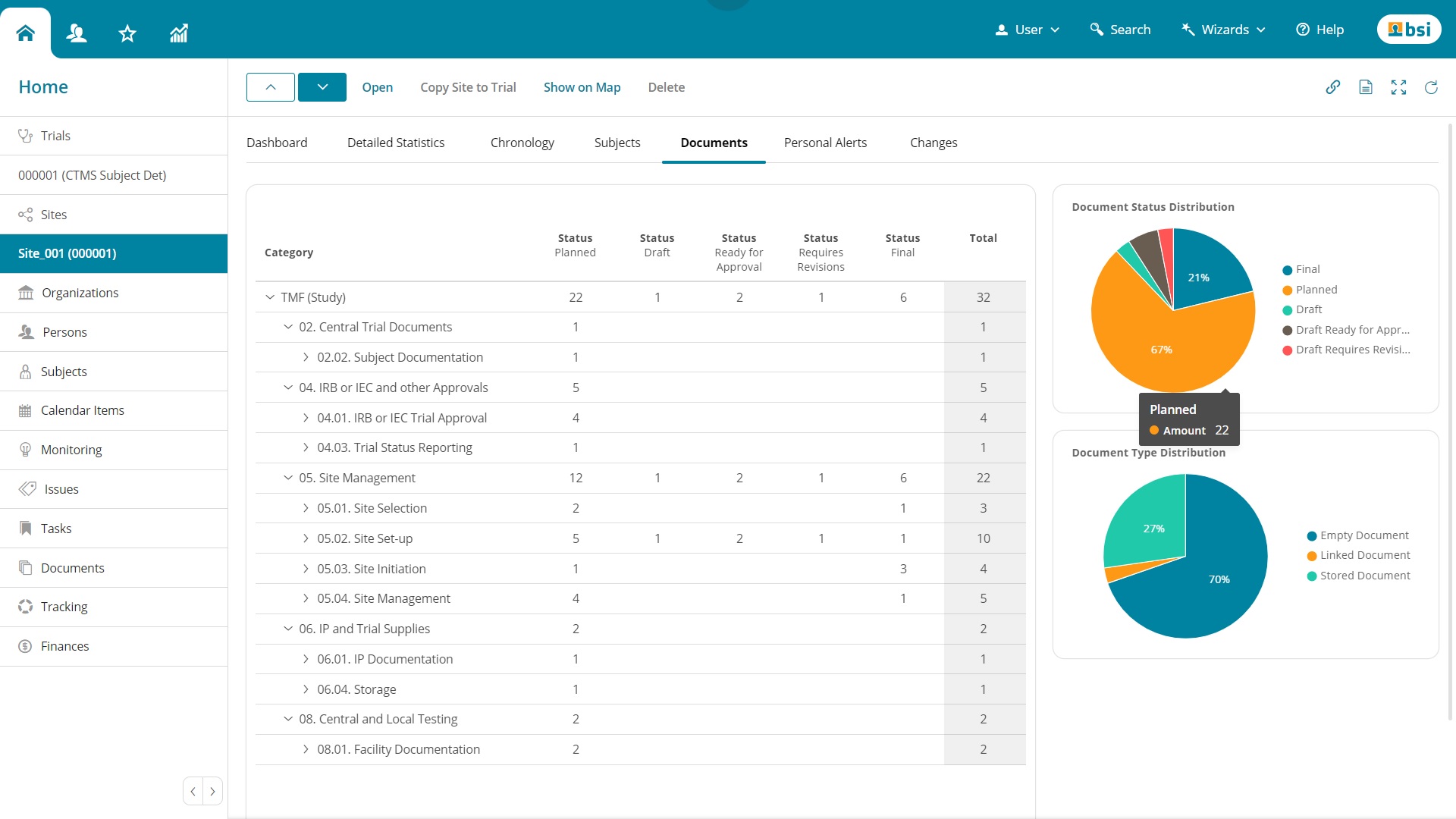
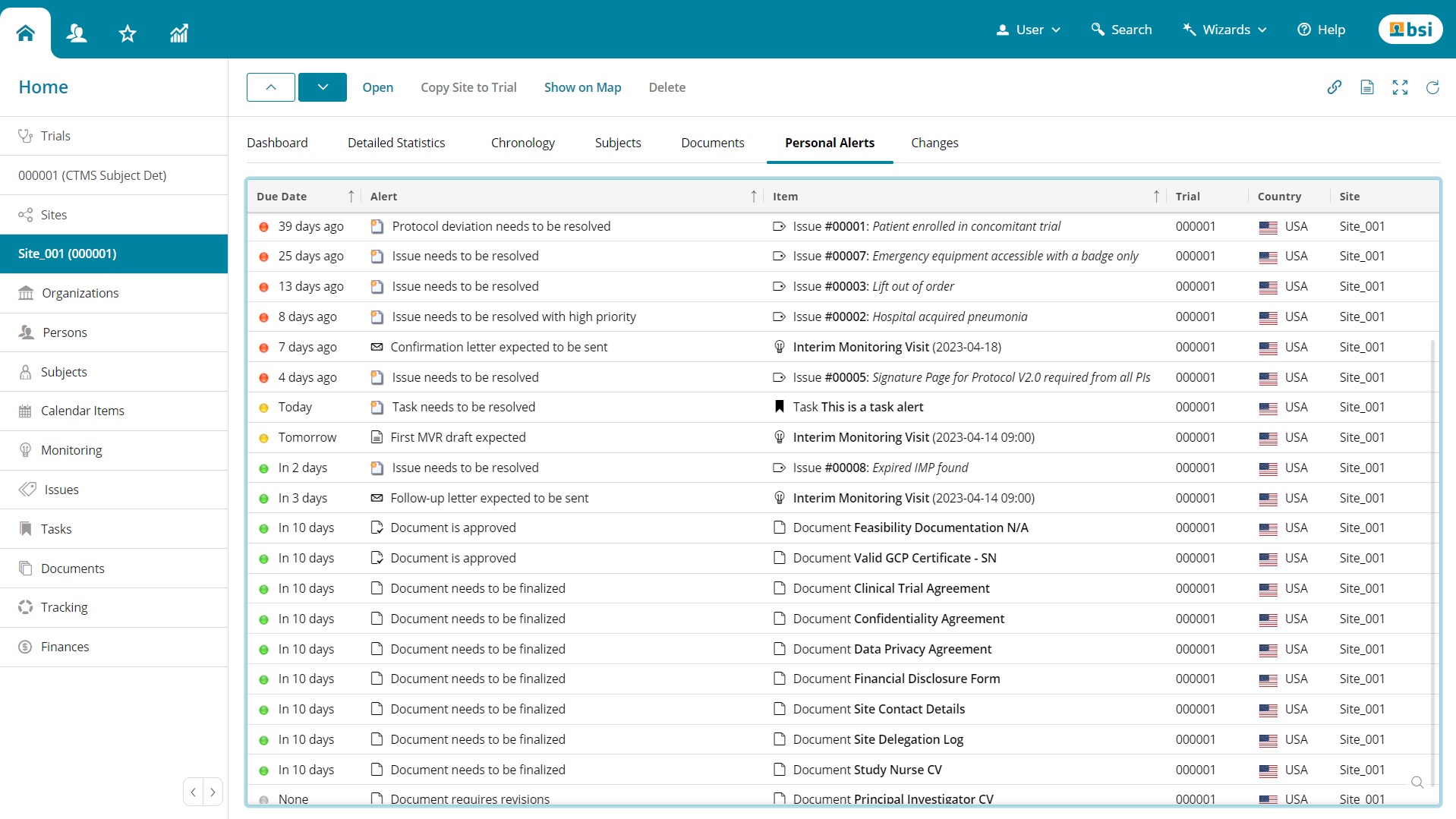
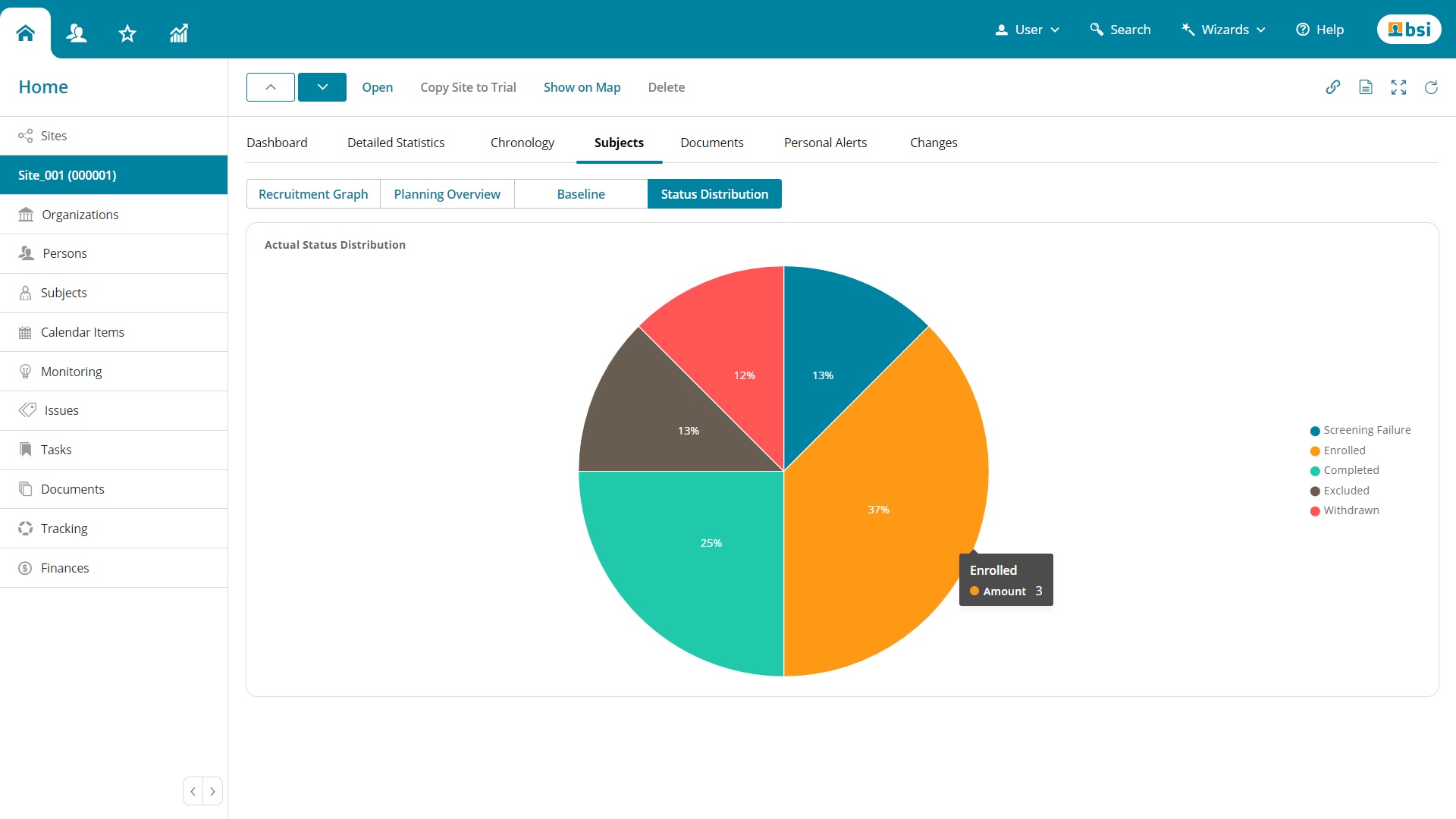
Categories
Features
FAQ
The official website of BSI CTMS is https://www.bsi-lifesciences.com/solutions/clinical-trial-management-system-ctms
BSI CTMS is a state-of-the-art Clinical Trial Management System with integrated eTMF, eMVR & TSM for Pharma, Biotech, MedTech, CROs & SMOs. A user-friendly interface with a hierarchical navigation tree allows you to oversee all aspects of your studies, from regulatory approvals to recruitment, from payments and contracts to monitoring and inventory. Plus, the mission control dashboard and alert notification system ensure you ll never miss a deadline! - 21 CFR Part 11 & EU Annex 11 compliant.
BSI CTMS belongs to the Clinical Trial Management category.
BSI CTMS offers features such as 21 CFR Part 11 Compliance, Audit Trail, Data Import/Export, Document Management, Patient Monitoring, Workflow Management.
No, BSI CTMS does not offer a free trial.
Reviews(0)
Write a reviewBSI CTMS alternatives

Smartsheet
Smartsheet helps small and midsize businesses manage projects, tasks, and workflows using a spreadsheet-style interface. Its most used by administrative and IT teams for daily collaboration and task tracking. Reviewers highlight its form creation and...load more

Castor EDC
Castor EDC is a comprehensive clinical research platform that enhances how clinical trials are run. It offers EDC, ePRO, and eConsent solutions, all aimed at improving data collection and clinical operations to accelerate the delivery of therapies, d...load more

Viedoc
Viedoc designs engaging software for the life science industry. By accelerating clinical trials on all levels, the Viedoc solutions support major pharmaceutical, biotech, and medical device companies, as well as renowned research institutions worldwi...load more

Visual Planning
Visual Planning is a project management tool that helps coordinate projects and resource schedules with calendars and rules. Its used mainly by small businesses across roles such as administrative and consulting in industries like accounting, healthc...load more

Florence eBinders
Digitize all of your study binder workflows with Florence eBinders and provide remote access for start-up, monitoring, and source data review for your sponsors. eBinders is trusted by 18,000+ research sites around the globe. Create, edit, distribute,...load more

RealTime-CTMS
RealTime eClinical Solutions simplifies the operations and management of clinical trials with a customizable eClinical system.

Medrio
Medrio empowers faster, higher-quality clinical trials with greater control. With almost two decades of experience, Medrio delivers proven, scalable solutions, unrivaled customer support, and guidance to industry innovators. Our suite of solutions, i...load more

Propel
Propel is a cloud-based solution that helps businesses handle PLM, QMS, PIM, and supplier management natively on the Salesforce app.

ShareCRF
Managing clinical trial data doesn t have to be complicated. What if you could set up your study in hours, without coding or waiting for external help? With an intuitive interface, ShareCRF puts you ... Read more

OpenClinica
Offering leading biotech and pharmaceutical companies, CROs, academic institutions, and government agencies the foundational clinical data management tools they need (EDC, ePRO/eCOA, RTSM, and reporting) with innovative, next-generation technology (E...load more